electron dot structure|7.3 Lewis Symbols and Structures : Tuguegarao Learn how to draw Lewis structure, also known as electron dot structure, of atoms, ions, and molecules. Follow the steps and rules using the octet rule, formal charge, and bond types. Dusit Thani is a perfect & intimate wedding venue that incorporates elegance & sophistication into one frame. Make your special day unforgettable. . Hotel Guests Rooms -+ Adults -+ Children -+ Promo When do you arrive? . 1223 Makati City Metro Manila, Philippines, () , ,
[email protected]; Call +63 (2) 7238 8888 .
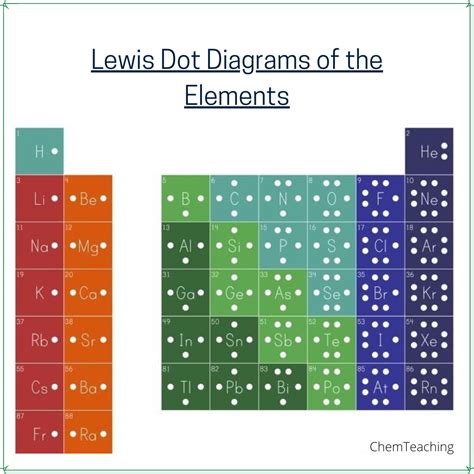
electron dot structure,Hun 27, 2022 — Learn how to draw Lewis electron dot diagrams for atoms and ions using dots around the symbol of the element. See examples, exercises and explanations for different valence electron configurations and periodic trends.The drawing of Lewis electron-dot structures is guided largely by the octet .7.3 Lewis Symbols and Structures Okt 11, 2017 — Learn how to draw Lewis dot structures or electron dot structures to represent the bonding and lone pairs of atoms in molecules. See examples, .Lewis structures – also called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs) – are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds. The Lewis structure wa.
Learn how to draw Lewis structure, also known as electron dot structure, of atoms, ions, and molecules. Follow the steps and rules using the octet rule, formal charge, and bond types.Learn how to draw Lewis electron dot diagrams for atoms and monatomic ions with less than 20 atomic number. See examples of covalent bonding and Lewis structures for nonmetal elements.Learn how to write Lewis symbols for neutral atoms and ions using dots to represent valence electrons. See examples, exercises and key takeaways for this topic.Ene 31, 2024 — The drawing of Lewis electron-dot structures is guided largely by the octet rule: that atoms form bonds to achieve eight electrons in their valence shell. For many elements, a .Okt 19, 2013 — Learn how to draw dot structures for molecules using simple rules and examples. Khan Academy offers free, world-class education for anyone, anywhere.Learn how to draw lewis dot structures, also known as electron dot structures, for elements and compounds. Find out how to use valence electrons, octet rule, and group numbers to create lewis structures.

The web page for Lewis symbols and structures in OpenStax Chemistry 2e is not working properly. It shows an error message and asks to restart the browser or visit the support center.Dis 7, 2019 — Steps to Drawing a Lewis Structure . Pick a central atom. Start your structure by picking a central atom and writing its element symbol. This will be the atom with the lowest electronegativity.Sometimes it's difficult to know which atom is the least electronegative, but you can use the periodic table trends to help you out. Electronegativity typically increases as you .
electron dot structure 7.3 Lewis Symbols and Structures Okt 19, 2013 — Learn how to draw dot structures for molecules using simple rules and examples. Khan Academy offers free, world-class education for anyone, anywhere.

Lewis structures (also known as Lewis dot diagrams, electron dot diagrams,"Lewis Dot formula" Lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the .Okt 18, 2015 — Finally, you'll understand all those weird pictures of molecules with the letters and the lines and the dots! Those are lewis dot structures. Let's learn how.
These structures, also known as lewis structures or electron dot structures, are drawings that visually demonstrate how electrons are shared and arranged around atoms. The electrons denoted as dots are called lone pairs and belong to an individual atom. . What is the lewis dot structure of carbon tetrachloride (CCl 4)? There are 32 valence .Ene 23, 2023 — An electron is represnted as a dot. A bond, which is made up of 2 shared electrons, is represented by two dots between the bonded atoms or a line . However, quantum mechanical electronic structure calculations find little d-orbital participation in the bonding, as would be expected for expanded octet bonding, suggesting that the formal charge .
Figure 7.10 Cations are formed when atoms lose electrons, represented by fewer Lewis dots, whereas anions are formed by atoms gaining electrons. The total number of electrons does not change. . To draw the Lewis structure for an odd-electron molecule like NO, we follow the same five steps we would for other molecules, but with a few minor .Electrons that are not in the valence level are not shown in the Lewis symbol. The reason for this is that the chemical reactivity of an atom of the element is solely determined by the number of its valence electrons, and not its inner electrons.Lewis symbols for atoms are combined to write Lewis structures for compounds or molecules with bonds between atoms.Ago 15, 2018 — The structure in the figure above is much more helpful – we see how the different atoms are connected together to form the molecule. Lewis Electron-Dot Structures. In a previous chapter, you learned that the valence electrons of an atom can be shown in a simple way with an electron dot diagram. A hydrogen atom is shown as H• because of its .The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has .Lewis structure, also known as Lewis dot structure or electron dot structure, is a simple and straightforward way of representing the outermost electron shell in a chemical species like an atom, ion, or molecule. It shows how electrons are positioned around the atoms either as lone pairs or in a chemical bond, typically a covalent bond or a .
Ene 31, 2024 — Figure \(\PageIndex{1}\): Lewis dot structure of atoms comprising ammonia and the dot structure of the molecule. (Kathryn Haas; CC-NC-BY-SA) The drawing of Lewis electron-dot structures is guided largely by the octet rule: that atoms form bonds to achieve eight electrons in their valence shell. For many elements, a full valence shell has an .electron dot structureGilbert N. Lewis introduced a diagrammatic system to represent the valence shell electrons in an atom. It helps us a lot while doing chemical bonding. He used dots to represent the valence electrons. He used lines to indicate bond .
Dis 20, 2023 — Lewis Electron dot Structure is a type of representation of valence electrons of atoms and molecules and compounds with their bond structure. Atoms are the tiny particles of an element that are responsible for chemical reactions whereas, molecules are groups of atoms that are chemically bonded together.
Ago 15, 2020 — Figure \(\PageIndex{1}\): Lewis dot structure of atoms comprising ammonia and the dot structure of the molecule. (Kathryn Haas; CC-NC-BY-SA) The drawing of Lewis electron-dot structures is guided largely by the octet rule: that atoms form bonds to achieve eight electrons in their valence shell. For many elements, a full valence shell has an .
Dis 29, 2020 — Here are the steps to draw a Lewis structure. The example is for the nitrate ion. A Lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons.The diagram is also called a Lewis dot diagram, Lewis dot formula, or electron dot diagram.Okt 16, 2019 — How It Works . A Lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell.As an example, an oxygen atom has six electrons in its outer shell. In a Lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons.GENERAL TERMS FOR LEWIS DOT STRUCTURES: 1. Dot • one dot represents one valence electron (found on odd-electron particles). 2. Pair of Dots •• a pair of dots represents a nonbonding (lone) pair of electrons that are not involved in a covalent bond and "belong to" only one atom. 3. Dash each dash represents two electrons that are shared between two atoms as .
electron dot structure|7.3 Lewis Symbols and Structures
PH0 · Lewis structure
PH1 · Lewis Electron Dot Structures
PH2 · Lewis Dot Structures
PH3 · Lewis Dot Structure: Definition, Examples, and Drawing
PH4 · Khan Academy
PH5 · 9.2: Lewis Electron Dot Diagrams
PH6 · 7.3 Lewis Symbols and Structures
PH7 · 7.2 Lewis Dot Structures – Introduction to Chemistry
PH8 · 6.1 Lewis Electron Dot Symbols
PH9 · 3.1: Lewis Electron